Promotions
LIGHTHOUSE® Emergency Kits
Be Prepared to save a life with our LIGHTHOUSE® Medical Emergency kits. LIGHTHOUSE® Emergency kits are designed to assist clinics in overcoming the most common medical emergencies that could occur in your dental office.
Explore the additional offerings that HANSAmed provide with LIGHTHOUSE® Emergency kits: Automatic Replenishment Service, Annual Subscription Service, Online Training, Drug Disposal Service, AEDs and individual medications.
Please note that the market is currently experiencing a shortage of Allerject® Autoinjectors. For this reason, all kits that contain Allerject® Autoinjectors are temporarily unavailable. We will update our website as soon as they become available again.
$$ Lighthouse, Lighthouse Kit, Light, Lig, Lighthouse, Emergency kit, medical emergency, basic kit, standard kit, deluxe kit, emer, emergency, kit, light, lighouse lifeline emergency kits for dental offices, dental emergency preparedness, Lighthouse emergency kits, dental emergency supplies, dental office safety kits, emergency medical supplies for dentists$$Geistlich Bio-Oss® & Bio-Oss® Pen
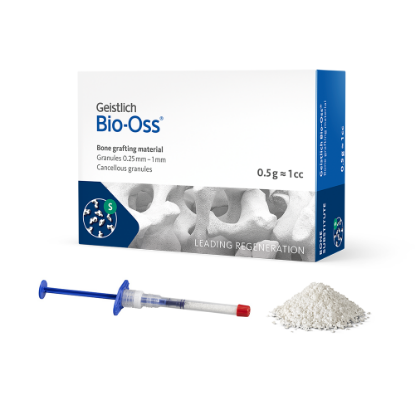
Geistlich Bio-Oss® is a sterile, biocompatible porous bone mineral substitute, which is physically and chemically comparable to the mineralized matrix of human bone. It is available in spongiosa (cancellous granules) in vials or Geistlich Bio-Oss Pen® pre-filled syringes.
$$ Bio, bios, biooos, biooss, bio-oss, bio oss, pen, geistlich, Bio-oss pen, bone grafting material, dental biomaterial, bios pen, bio oss pen, oss pen creo xenogain, MinerOss, MinerOss XP, MinerOss X, CeraOss and SynMax , advance medical solutions, Xenogenic Bone Graft, biosurgicals, inteross Geistlich Bio-Oss, Bio-Oss Pen, bone augmentation, Geistlich, bone grafting material, dental biomaterials$$Geistlich Bio-Oss® Collagen
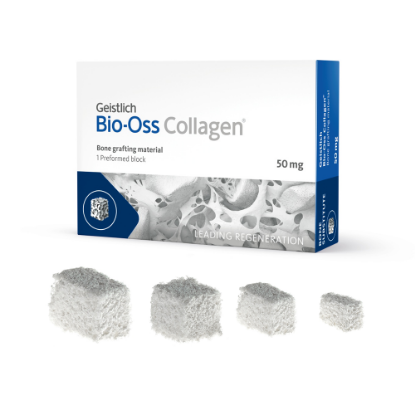
Geistlich Bio-Oss® Collagen consists of 90% Geistlich Bio-Oss® granules and 10% integrated porcine collagen. Geistlich Bio-Oss® is scientifically proven and is the leading biomaterial in regenerative dentistry. Combining Geistlich Bio-Oss® with highly purified porcine collagen enhances it’s handling characteristics making Geistlich Bio-Oss® Collagen formable and easy to handle.
$$ Bio, bios, biooos, biooss, bio-oss, bio oss, collagen, geistlich, Bio-oss collagen, colagen, dental implant, bone augmentation, guided bone regeneration, GBR, bone graft material, collagen matrix creo xenogain, MinerOss, MinerOss XP, MinerOss X, CeraOss and SynMax , advance medical solutions, Xenogenic Bone Graft, biosurgicals Geistlich Bio-Oss Collagen, dental bone graft, bone regeneration, oral surger, dental implants, bone augmentation, biomaterials, Geistlich, osteoconductive matrix, guided bone regeneration, GBR, bone graft material, collagen matrix$$Geistlich Fibro-Gide®
Geistlich Fibro-Gide® is a porcine, porous, resorbable and volume-stable collagen matrix, specifically designed for soft-tissue regeneration.
$$ fibro, fibrogide, fibro-, fibro-gide, fibro-guide, fibro guide, dental implant, collagen matrix, dental biomaterials, tissue augmentation, geist, geistlich, soft tisue grafting Collaguide Membrane, zcore form Geistlich Fibro-Gide, soft tissue volume augmentation, oral surgery, dental implants, collagen matrix, soft tissue grafting, Geistlich, dental biomaterials, mucogingival surgery, tissue augmentation, gingival recession treatment$$Geistlich Mucograft®
Geistlich Mucograft® is a unique 3D collagen matrix designed specifically for soft tissue regeneration. It is indicated for the gain of keratinized tissue and for recession coverage.
$$ muco, muc, mucograf, mucograft, micograft, soft tissue regeration, geistlich, biomaterials, geist, collagen matrix, geistlich mucograft, matrices nobel biocare, henry schein, cytoplast Geistlich Mucograft, soft tissue regeneration, oral surgery, dental implants, periodontal therapy, collagen matrix, Geistlich, mucogingival surgery, dental biomaterials, tissue augmentation, gingival recession$$Neomem® FlexPlus
Philips HeartStart Onsite AED
The Philips HeartStart Onsite AED is one of the most popular automated external defibrillators. The Philips HeartStart Onsite was carefully designed for first responders and health care professionals with a need for an AED program. With the combination of technology and design in mind, Philips has significantly upped the ante for AEDs with their HeartStart Onsite. The Philips HeartStart Onsite is Philips' flagship AED within their HeartStart lineup. The Philips HeartStart Onsite is durable, small, light, and versatile.
$$ filips, phil, heart, aed, defi, defrib, emergency, eme, emer Zoll AED Plus, lifeline Philips HeartStart OnSite AED, automated external defibrillator, Philips AED, emergency defibrillator$$Ultracaine®
Ultracaine® D-S Regular & Ultracaine® D-S Forte
Ultracaine® is the world’s original articaine and is exclusively available through HANSAmed.
$$ ulta, anesthesia, anaesthesia, anes, pain, pain control, articaine, articanie Cookwaite, Anestaject, septanest, septodont, xylocaine, orabloc Discover Ultracaine - a fast-acting dental anesthetic for effective pain management during dental procedures. Ideal for tooth extractions, oral surgery, and ensuring patient comfort with quick onset and reliable performance.$$Wound Dressings
Cook-Waite
Cook-Waite Lidocaine HCl 2% w/ Epi 1:100,000 (Red) contains 2% Lidocaine HCl injection with 1:100,000 epinephrine bitartrate.
Cook-Waite Lidocaine HCl 2% w/ Epi 1:50,000 (Green) contains 2% Lidocaine HCl injection with 1:50,000 epinephrine bitartrate.
$$ cook, cok, cock, cookwaite, cook-waite, anesthesia, dental anesthesia, anest, anestesia Ultracaine, articaine, septanest, xylocaine Explore Cook-Waite products. Cook-Waite offers quality dental anesthesia for effective patient care.$$GEM21S:Growth-Factor Enhanced Matrix Kit 0.5cc BTCP/0.5 ml rhPDGF, 1cup and 1 vial/kit
GEM 21S® is a growth-factor enhanced matrix that plays a critical role in promoting rapid bone and soft tissue regeneration in oral surgery. It contains purified recombinant platelet-derived growth factor (rhPDGF), which significantly accelerates the body's natural healing process.
* Flat $50 shipping fee per order, regardless of the number of boxes purchased, due to cold chain requirements.
**GEM 21S® must be kept refrigerated. Due to this storage requirement, returns cannot be accepted.
Neoderm™ ADM
PeriAcryl®
PreservaRidge™ Plug
SciCan OPTIM 33TB Surface Disinfectant
OPTIM has demonstrated effectiveness against viruses similar to Coronavirus Disease 2019 (COVID-19) on hard non-porous surfaces and non-invasive medical devices. This product can therefore be used against COVID-19 when used in accordance with the directions for use against Poliovirus on hard, non-porous surfaces, and non-invasive medical devices.
$$ SciCan, OPTIM, optim 33TB, Surface Disinfectant, Disinfectant, wipes, cleaner caviwipes, cavicide dexidin, henry shcein, nobel care SciCan Optim 33TB surface disinfectant, surface disinfection, medical disinfectant solution$$