Membranes & Matrices
Choose from a full range of membranes and matrices to support the most complex grafting needs!
Features & Benefits
The OpenTex® TR Membrane may be shaped or cut to your preferred size.
OpenTex® TR membrane and the titanium can be trimmed to the required size.
It will be important to verify that the edges of the titanium are smooth after trimming them. You want to make sure that there are no sharp edges so it as to not puncture through the flap of tissue.
Features of OpenTex®
Unlike traditional membranes, the soft tissue attaches, but does not grow through the membrane with OpenTex®.
The exposed membrane also allows for non-surgical removal therefore no anesthesia is required.
Why you should use a barrier membrane
Neomem® acts as a barrier to prevent the epithelial cells from growing into the bone graft. By not placing a membrane, your bone graft can be exposed to tissue growth, bacteria and food.
Applications
Suturing technique for socket preservation using Neomem® Xac.
There are many different suturing techniques. We cannot recommend one over the other. However, in socket preservation procedures, it seems as if many clinicians seem to prefer a reverse figure eight type of suture technique. The reverse technique refers to the movement of the suture needle from inside the socket to the outside. This aids in membrane retention. In terms of suture material Biotex PTFE (4.0 or 5.0) is mostly used due to the monofilament nature, which eliminates bacterial wicking.
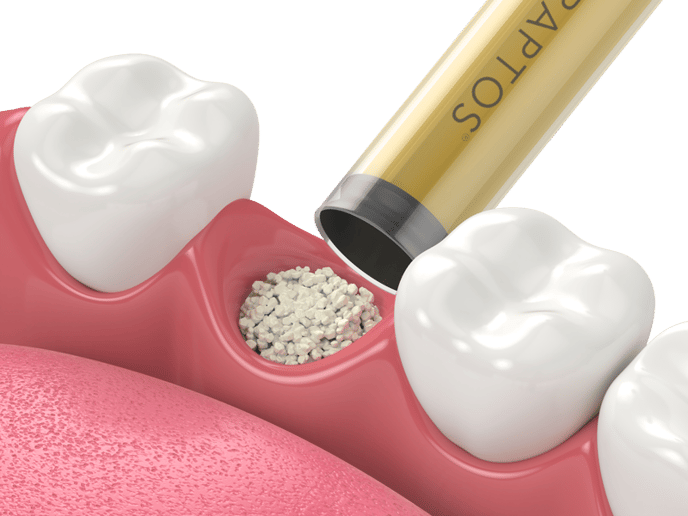 | 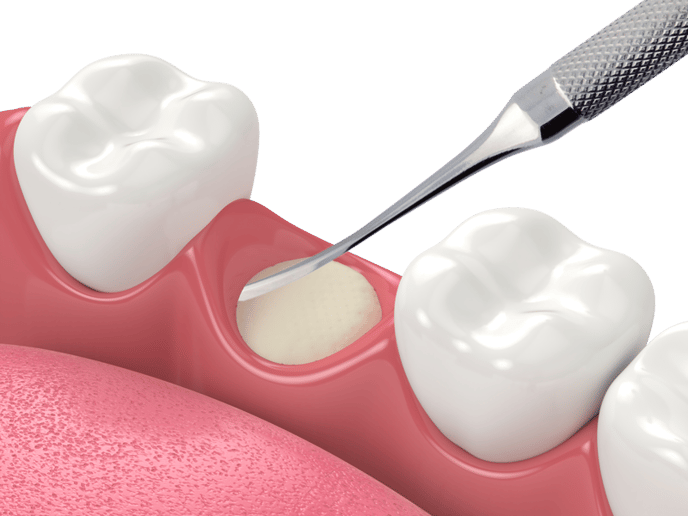 | 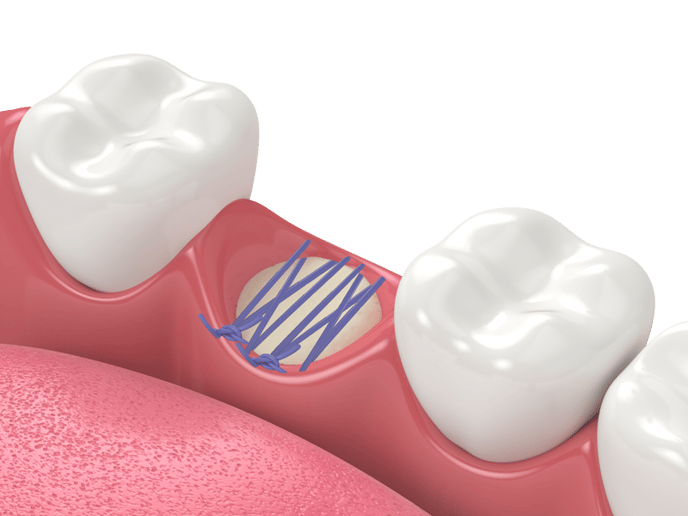 |
| Following a tooth extraction, fill the socket using bone particulates such as Raptos®?Allograft, to prevent bone loss, maintain position of adjacent teeth and allow a healthy bone ridge for future implant support. | To protect your grafting site, place a dry, untrimmed 12 x 12?mm Neomem®?Xac (on any side) over your graft using dry forceps. Neomem®?Xac will quickly rehydrate and adhere to the surgical site. Then use a wet membrane placement instrument such as our MPI-1 to hydrate the membrane and tuck the edges as needed. | Using a PTFE suture such as Biotex™, suture using a reverse figure eight type technique (from inside the socket to outside) to fold down the edges of the soft tissue to better hold in place the membrane and protect the graft. Since PTFE sutures are non resorbable and need to be removed, a longer lasting resorbable monofilament suture, such as Monoglyc™, can be used as an alternative to PTFE sutures. |
TIP
Since PTFE sutures are non resorbable and need to be removed, a longer lasting resorbable monofilament suture, such as Monoglyc™, can be used as an alternative to PTFE sutures.
When Free Gingival Grafts (FTG) or Connective Tissue Grafts (CTG) aren't available, an ADM can be an option.
ADMs have been used for gingival recession situations where primary, relaxed flap coverage can be obtained to progressively build up the underlying and adjacent tissue. If you have a situation where the potential harvest site has insufficient tissue to provide adequate harvest volume, then an option exists in the form of acellular dermal matrices or ADMs. These are also referred to as acellular dermal membranes, and are identified in the marketplace as either ADMs or ACDs. Our version is known as Neoderm ADM. Neoderm ADM is decellularized to remove cellular components and to preserve the biologic properties that promote revascularization and repair.
Alternatives when you cannot use your patient's soft tissue.
Neoderm ADM (an Acellular Dermal Matrix) can be used in areas where root coverage is required in order to gain keratinized tissue to thicken native tissue. When using Neoderm ADM, it is best to get as much coverage with native tissue as possible in order to reduce the effects of exposure to get a predictable result.
Acellular Dermal Matrix (ADM) is a soft connective tissue graft generated by a decellularization process that preserves the intact extracellular skin matrix. Upon implantation, this structure serves as a scaffold for donor-side cells to facilitate subsequent incorporation and revascularization.
A solution for when a secondary surgical site is not an option.
Yes, Neoderm ADM (an Acellular Human Dermis Membrane), can be used in areas where root coverage is required in order to gain keratinized tissue and to promote attached gingiva; also to repair recession defects or to thicken native tissue. As an ADM, it is desired to get as much coverage with native tissue as possible in order to reduce the effects of exposure to get a predictable result.
Acellular dermal matrix (ADM) is a soft connective tissue graft generated by a decellularization process that preserves the intact extracellular skin matrix. Upon implantation, this structure serves as a scaffold for donor-side cells to facilitate subsequent incorporation and revascularization.
Product characteristics
Positioning of the PTFE OpenTex® and OpenTex® TR membranes
The regular OpenTex® membranes can be placed either side up but for the Titanium reinforced version, OpenTex® TR, it is preferable to place down the dark side of the titanium over the bone.
The Neomem® Xac manufacturing process
The process to manufacture Neomem® Xac is centered on tightly controlled procedures focused on:
- Best in class procedure, driven by internal research and peer reviewed studies
- Minimal manipulation to retain optimal regenerative capacity native to tissue
- Exhaustive post-processing rinsing to effectively eliminate processing reagents
Independent, contracted tissue recovery agencies follow strict protocols in accordance with FDA regulations and AATB standards. Serological and microbiological testing is performed at an independent, CLIA certified, FDA registered laboratory against standards that exceed the requirements of the FDA and AATB. Final donor eligibility is determined by a licensed medical director following review of all infectious disease testing, the uniform donor risk assessment interview and all relevant medical records.
Placental membranes are recovered from cesarean section procedures and shipped to the manufacturer overnight in validated coolers. The membranes are processed fresh upon receipt and never frozen. The membrane is subjected to a proprietary series of mild aqueous rinses to disinfect and clean the tissue in ISO Class 6 clean rooms without the use of harsh chemicals. Once rinsed, the tissue is dehydrated, cut to size, packaged in foil and tyvek pouches. Finished grafts are evaluated by dedicated quality control technicians and subjected to terminal sterilization via low dose e-beam irradiation to a sterility assurance level of 10-6.
Every step of the process is completed in accordance with current Good Tissue Practices (cGTP), AATB standards and AORN aseptic technique. All processes are validated against appropriate ISO standards.
Finally, a comprehensive review of the infectious disease testing, processing records, labeling documentation, and medical records are completed by a dedicated quality associate prior to the tissue being released for distribution.
Is there an orientation to Neomem® Xac?
No, there is no orientation. Neomem® Xac amnion-chorion membrane, as well as all others we have seen in dentistry, do NOT have any orientation, as they are deepithelialized. They can be placed up or down, folded, and/or layered, without any concern.
Amnion-chorion membranes generally resorb in 8 to 12 weeks. If the membranes are left exposed, this absorption profile will obviously differ, but given the many factors that could impact an exposed membrane, it is not possible to give a precise resorption profile for an exposed membrane. However, Neomem® Xac amnion-chorion membranes have proven to behave exceptionally well under exposure.
Product comparisons
Comparison between Neomem® and Neomem® Flexplus
The main difference between the two is that one is derived from highly purified type I collagen fibres from bovine Achilles tendon.
The other is a single layer collagen membrane derived from porcine peritoneum. Neomem® may be too stiff in some instances, with too much memory that causes it to spring back. Neomem® FlexPlus is supple and drapy ensuring that it drapes over the ridge easily.
Benefits of using Neomem® FlexPlus
Neomem® Flexplus is easier to use, there is no right or wrong side, it causes less inflammation and Neomem® Flexplus is three times stronger than Bio-Gide®¹.
1. Li ST, Yuen D, Martin D, Shishido Lee N. 2015. A Comparative Study of a New Porcine Collagen Membrane to Bio-Gide®. Science, Technology, Innovation. Data on file.
All trademarks are the property of their respective owners.
General definitions
Neoderm ADM and the definition of ADM
The acronym stands for Acellular Dermal Matrix, also referred to as Acellular Dermal Membranes.
Acellular dermal matrix (ADM) is a soft connective tissue graft generated by a decellularization process that preserves the intact extracellular skin matrix. Upon implantation, this structure serves as a scaffold for donor-side cells to facilitate subsequent incorporation and revascularization.
Our version is known as Neoderm ADM.
Definitions of different soft tissue grafts.
Connective Tissue Grafts (CTG) are most commonly harvested from the palate or tuberosity composed of the inner layer of connective tissue below the gingival tissue. Free Gingival Grafts (FGG) are harvested from the palate or tuberosity as a full thickness graft involving the gingival tissue and underlying connective tissue.
If you have a situation where the potential harvest site has insufficient tissue to provide adequate harvest volume, then an option exists in the form of Acellular Dermal Matrices or ADMs. Our version is known as Neoderm ADM.
Safety information
Guidelines after using Neomem® Xac
If the membrane is exposed to the oral environment, then for 3 days post-op please instruct the patient to avoid overly aggressive rinsing or swishing with any solution to avoid dislodgement of the membrane.
After 3 days, gentle rinsing with salt water is recommended for the next 7 days.
The patient should not use any oral rinses 10 days post-op. This includes antiseptics; chlorhexidine (Peridex®), chlorhexidine without alcohol, and other common oral rinses. Oral rinses can adversely impact the health of gingival cells, and chlorhexidine specifically may adversely impact the membrane. After 10 days post-op, the patient may begin using an oral rinse for plaque control.
Safety information on our Acellular Human Dermis Membrane.
It is important to note that all tissues have been collected, processed, stored and distributed according to the Current Standards for Tissue Banking of the American Association of Tissue Banks (AATB), and Food and Drug Administration (FDA) Regulations and Ministry of Food and Drug Safety (MFDS) Regulations.
Neoderm ADM is decellularized to remove cellular components and to preserve the biologic properties that promote revascularization and repair. It is minimally processed to remove epidermal and dermal cells, while preserving the extracellular matrix of the dermis. Neoderm ADM is sterilized with ethylene oxide gas (EO gas) after processing and packaging.
Composition and origin of Acellular Dermal Matrices.
The most common sites of harvest for dermis grafts are the buttocks and inner thigh or back. The dermis is a connective tissue layer sandwiched between the epidermis and subcutaneous tissue. The dermis is a fibrous structure composed of collagen, elastic tissue, and other extracellular components. A dermis graft is a surgical procedure in which a piece of dermis is transplanted from a deceased human donor, processed and transplanted to another.
Acellular dermal matrix (ADM) is a soft connective tissue graft generated by a decellularization process that preserves the intact extracellular skin matrix. Upon implantation, this structure serves as a scaffold for donor-side cells to facilitate subsequent incorporation and revascularization.
Our version is known as Neoderm ADM.
Things to know and steps to follow before using Neoderm ADM.
It is quite easy to prepare Neoderm ADM for use, there are just a few steps to follow one by one. Warm saline solution(37°C) may reduce the time required for rehydration. Nevertheless, do not heat saline above 37°C. When rehydrating multiple pieces, ensure the pieces are not overlapping or clumping together as this may delay the process. Use multiple dishes, if necessary. Fully submerge Neoderm ADM by weighing it down, e.g., with sterile forceps. Using a sterile gloved hand or forceps, remove and discard the paper backing (the 10x10 size is backing free) once it separates from the tissue. Neoderm ADM is fully rehydrated when it is soft and pliable throughout. Neoderm ADM will appear to be of uneven thickness and have a mottled appearance if it is not fully rehydrated. Once Neoderm ADM is removed from the packaging, it should be rehydrated within 24 hours and can be stored up to 4 hours prior to use. Neoderm ADM has an up/down side. The down side (dermal) has a paper backing meant to protect the absorbent characteristics of the dermal matrix. After hydrating for at least 10 minutes in sterile saline, remove the paper backing and place this dermal side onto the wound bed. You should see a rapid absorption of blood from the wound bed into the dermal side as an indication that the correct side is in the ideal orientation.


